Products that are sensitive to contamination must be manufactured in a way that meets exacting standards of efficacy and quality. All quality aspects are considered in relation to the risks of the route of administration: injectable, oral, and so on; and the way they are produced: disinfected, terminally sterilized, or under less controlled conditions. In this article from Ekeas cleanroom, we will review cleanroom standards and their classifications.
Federal Standard 209 Cleanroom
The first federal standard for classifying cleanrooms was Federal Standard 209, published in 1963, titled “Cleanroom and Work Station Requirements, Controlled Environments.” This standard was later revised in 1966 (FS209A), 1973 (B), 1987 (C), 1988 (D), and finally in 1992 (E), which included the conversion of the cleanroom class units to metric. In addition, this standard provides information on the type of equipment required to test areas along with information on the location of samples, the sample volume required to achieve statistical significance, and the maximum particle concentration at each specific location. This standard was based on 0.5-micron particles, although smaller measurements were also possible (laser particle counters of 0.1-microns were available from 1972), but this was the only size that was repeatable with reliability.
Depending on the cleanliness class of the cleanroom and the design of the cleanroom, one of several methods of sampling was used. This was also to ensure that both the amount of particles in the air was less than the permissible limit and that it was uniform throughout the cleanroom.
read more | Cleanroom Validation
The Federal Standard became a de facto standard for classifying cleanrooms and is still used by some to express the class of cleanrooms. Class 100 cleanrooms, class 10,000 and so on are common terms used to classify cleanrooms, although since 1999, the Federal Standard is no longer the prevailing standard. An ISO standard was published for the description and classification of cleanrooms: ISO 14644. European countries adopted this standard in 1999 and Federal standards no longer recognized FS209E from 2002.
ISO 14644 cleanroom standard
The ISO 14644 standard consists of several subcategories, which are shown in the following table:
Constituent parts of ISO 14644
| Reference | Title | Review year |
| ISO 14644-1 | Cleanrooms and related controlled environments, Part I: Classification of air cleanliness | 1999 |
| ISO 14644-2 | Cleanrooms and related controlled environments, Part II: Description of tests and monitoring to demonstrate continued compliance with ISO 14644-1 | 2000 |
| ISO 14644-3 | Cleanrooms and related controlled environments, Part III: Measurement and test methods | 2005 |
| ISO 14644-4 | Cleanrooms and Related Controlled Environments, Part IV: Cleanroom Design, Construction and Operation | 2001 |
| ISO 14644-5 | Cleanrooms and related controlled environments, Part V: Operations | 2004 |
| ISO 14644-6 | Cleanrooms and related controlled environments, Part VI: Terms and definitions | 2007 |
| ISO 14644-7 | Cleanrooms and related controlled environments, Part VII: Advanced clean equipment | 2004 |
| ISO 14644-8 | Cleanrooms and Related Controlled Environments, Part VIII: Classification of Airborne Molecular Contaminants | 2006 |
| ISO 14644-9 | Cleanrooms and Related Controlled Environments, Part IX: Classification of Cleanliness of Surfaces by Particulate Matter | 2008 |
| ISO 14698-1 | Cleanrooms and Related Controlled Environments, Biological Contaminant Control, Part I: General Principles and Practices | 2003 |
| ISO 14698-2 | Cleanrooms and Related Controlled Environments, Biological Contaminant Control, Part II: Evaluation and Interpretation of Biological Contaminant Data | 2003 |
In the following, we will examine each of the ISO 14644 standard subsets:
A new set of standards for classifying and grading cleanrooms was adopted in 1999. The first of these was ISO 14644-1, which defined the classification of cleanrooms and the maximum number of particles allowed in a given volume of air. It is important to note that, while ISO 14644-1 is accepted worldwide for the classification of cleanrooms, there are differences in common monitoring methods, particularly between ISO 14644-1 and the EU GMPs and WHO guidelines.
Before testing, the condition in which the cleanroom certificate is to be issued must be specified. Three conditions are considered in the framework of ISO14644-1:
- As Built: A cleanroom in which all services are connected and ready for use but does not have production equipment or personnel.
- At rest: Conditions in which all services are connected, all equipment is installed and operating as agreed, but no personnel are present.
- In operation: Conditions in which all equipment is installed and operating as agreed, and a certain number of personnel are also present on site and working according to a specified procedure.
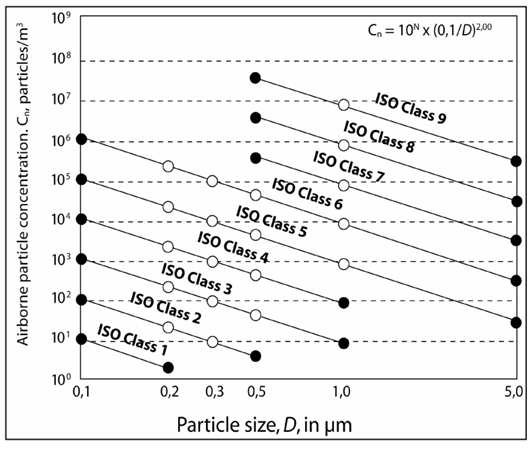
The limits are determined based on calculations related to the standard, in which the maximum concentration limit, particle size in micrometers, and its degree are classified. Therefore, the relationship between particle size and its frequency in a population is a function of , and if a particle size versus its concentration graph is plotted on a logarithmic/logarithmic scale, the slope of the curve for each group will be obtained as 08/2.
read more | Cleanroom Design
Graphical representation of air particulate concentration limits for specific grades of ISO standard classifications. [The horizontal axis of particle size in micrometers (D) and the vertical axis of the concentration of airborne particles (Cn), number in cubic meters]
To issue a cleanroom or clean area certificate, the following must be determined:
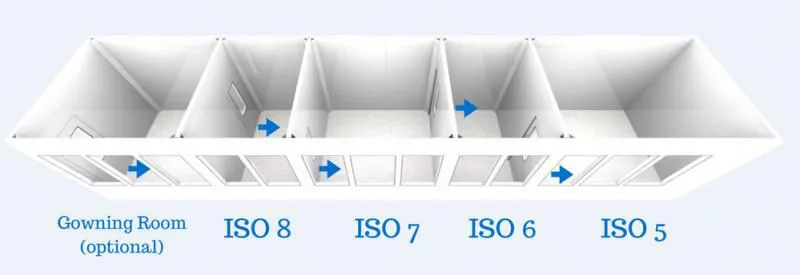
The class of the cleanroom should be expressed in the form of “ISO N degree”;
cleanroom occupancy status;
desired particle size. Cleanroom verification is also possible for multiple particles of different dimensions. In such a case, the sample volume should be considered proportional to the size of the largest particle.
ISO 14644-2 Cleanroom standard
Part 2 of the ISO 14644 standard was published in 2000 and became a complementary document to ISO 14644-1. This standard describes the frequency and requirements for demonstrating the continued compliance of a system to cleanroom classifications.
Once a cleanroom has been fully classified and its class has been determined, certain aspects must be maintained in order for the cleanroom user to still be able to claim that the cleanroom has maintained its compliance to the desired classification features and is in the same condition as when it was classified.
read more | Cleanroom Quality Documentation
The classification of a cleanroom must be repeated in accordance with the frequency defined in the ISO 14644-2 standard. For class 5 and cleaner classified rooms, this interval is every 6 months, and for class 6 or cleaner cleanrooms, it is every 12 months. This time interval can be extended to the maximum allowable range, provided that the organization concerned shows that “no significant changes” have occurred in the cleanroom control. Therefore, class 5 cleanrooms do not necessarily need to be re-validated every 6 months, and the reclassification period can be extended to a maximum of 12 months.
Recommended time intervals for reclassification.
| Classification | The longest period of time | Test Method |
| ISO 5 and less | 6 months | ISO 14644-1 |
| ISO 6 and more | 12 months | ISO 14644-1 |
This document also describes the risk assessment requirements that must be completed to determine the details of the monitoring plan. These include:
- What level of monitoring is required?
- What limits should be set for action and alert limits?
- What should be done if action or alert limits are exceeded?
In addition, the system used to apply control to the environment must be based on a written plan, and this plan must take into account the risks associated with the activities being performed and the best practices for monitoring the environment and establishing appropriate control limits.
Cleanroom standard ISO 14644-3 Cleanroom test methods
This section of ISO 14644 specifies the test methods for cleanrooms. Tests are specified for two types of cleanrooms: unidirectional flow and non-unidirectional flow (turbulent flow). The cleanroom test methods recommend what equipment and methods are required for testing and, if necessary, suggests an alternative method. For some tests, several different methods and devices are recommended.
read more | Hygienic air conditioner
A list of mandatory and optional tests and the relevant references is provided in the table below.
Test method requirements for classification of cleanrooms
| The relevant ISO standard | Items to be tested |
| 14644-1 , 14644-2 | Counting the number of dispersed particles for classification and test measurements of cleanrooms and clean air devices. |
| 14644-1 | Counting of very fine suspended particles |
| 14644-1 | Counting large suspended particles |
| 14644-1 و 14644-2 | Air flow test |
| 14644-1 و 14644-2 | Air pressure difference test |
| 14644-2 | Installed filter leakage test |
| 14644-2 | Testing and drawing the direction of the air flow |
| 7726 | Temperature test |
| 7726 | Moisture test |
| 14644-2 | Recovery test |
| 14644-1 و 14644-2 | Pollution leakage test |
ISO 14644-4 Cleanroom Standard: Design, Construction, and Start-up
This part of ISO 14644 specifies the requirements for the design and construction of cleanroom installations. It provides details of all the important elements of the design and construction of a cleanroom or clean facility.
Since this standard is focused solely on the design and construction of cleanrooms or clean facilities, it is necessary to mention other important aspects that are not covered in it, such as:
- Some specific functional aspects in relation to users
- The final application of the cleanroom
- Fire-fighting and safety regulations that are applied according to local regulations
- How to use the cleanroom in accordance with the operating environment
- Operation and maintenance of the cleanroom
This standard includes sections on defining requirements, planning and designing cleanrooms, construction and commissioning, testing and commissioning of the delivered facility, and documentation required for delivery of the facility to the end user.
ISO 14644-5 Cleanroom Operations
ISO 14644-5 provides a source of basic guidance on how to use a cleanroom and the operations that are essential for their use. These are: cleanroom clothing, personnel, fixed equipment, portable equipment and materials, and cleanroom cleaning and disinfection. This standard addresses these items in the form of cleanroom operational requirements that must be followed in this order or a procedure for each one should be considered. Then, it expands on them in each section to provide more information on how to implement each one. The following are addressed:
Cleanroom personnel: This section provides an overview of training for cleanroom users to ensure that they operate the cleanroom correctly. This section includes a sample cleanroom dress code and guidance on developing internal standards for health and safety.
Fixed equipment: This section focuses on how to arrange fixed equipment installed in the cleanroom, the parts of it that need to be cleaned, how to clean it, how to place it in the cleanroom, and how to transfer it out. It also describes how to perform preventive maintenance and how to do it while maintaining a controlled environment.
Portable materials and equipment: The transfer of materials can be considered the second-largest risk to a cleanroom. Therefore, controls should be in place to manage the transfer of contaminants into the cleanroom. This section covers many aspects of portable materials, including chemicals in piping, mops and cleaning buckets, notebooks and notepads, and by defining some criteria, it identifies features that reduce the risk.
Cleanroom cleaning: This section discusses the role of cleanroom personnel clothing in providing protection against the potentially harmful effects of cleanroom materials and in protecting clean products from personnel contamination. People are the primary source of contamination in a cleanroom, so it is very important to choose clothing to protect the environmental conditions and the product being produced. Clothing selection is based on three key factors: product sensitivity, cleanroom classification, and safety. This annex addresses the selection of clothing used and covers a range of clothing features available. Cleanroom cleaning requirements address methods, personnel, training, planning, and contamination surveys. This section describes routine cleaning methods and specific activities that may need to be controlled, but go beyond a routine activity, such as construction. This section defines a ten-step program for cleaning activities related to construction.
ISO 14644-6: Cleanroom Vocabulary
This part contains a comprehensive list of terms and definitions relevant to the ISO 14644 documents as well as the ISO 14698 series of documents for microbiological control of cleanrooms.
ISO 14644-7: Cleanroom Isolating Devices
This part of ISO 14644 (Part 7) specifies the minimum requirements for the design, construction, installation, testing and validation of isolating devices from aspects that differ from cleanrooms described in ISO 14644-4 and ISO 14644-5. Isolating devices can include both open and closed systems which include gloveboxes and rapid access containment systems.
This ISO document includes a set of annexes which provide additional information in relation to the ISO requirements. Annex A addresses the concept of isolation range. Based on this concept, a boundary can adequately separate internal and external conditions with a given volume.
read more | cleanroom equipment
ISO 14644-8: Classification of Airborne Molecular Contamination
This standard (Part 8) addresses the classification of airborne molecular contamination (AMC) in cleanroom air (CMA), for chemicals, and provides test, analysis and time-weighted factors for different classifications.
Global GMP requirements for pharmaceutical cleanrooms
European GMP
The European Commission’s guideline for sterile production was revised in 2003 to consolidate the various cleanroom standards and align them with the single ISO 14644-1 standard. The first page notes:
“The Commission Directive on Good Manufacturing Practice (GMP) is complemented by the Guide to the Application of the Principles and Guidelines of GMP for Sterile Medicinal Products. This Guide includes recommendations on environmental cleanliness standards for cleanrooms. This Guide has been reviewed in the light of the international standard and amended for alignment with it, but also takes into account specific concerns unique to the production of sterile medicinal products.”
In order to obtain certification, a cleanroom must comply with the rules and format of the ISO 14644-1 guideline, but this ISO standard was amended with regard to sterile medicinal products. For this purpose, a table of cleanroom certification values was defined that are roughly translated to ISO 14644.
Cleanrooms and clean air devices must first be classified and their class determined. They must then be monitored to ensure that conditions appropriate to the quality of the product are maintained. The data collected under this monitoring must be reviewed in the light of the risk that it poses to the quality of the final product.
**As shown above, the ranges in the EU GMP table are slightly different from ISO14644. To comply with the relevant certificate, it is important to be aware of the performance of ISO14644-1 and how to verify the cleanroom according to the standard rules on the number of sampling points, the location of the sampling point, and the sampling volume at each location. However, instead of using the ISO14644-1 classification range table, the table above, which is included in the revised guideline text, should be used.
GMP also defines other expectations, for example, the sample volume for A-class cleanrooms should be 1 cubic meter at each location, and due to the high deposition of 0.5 micrometer particles, the length of the pipe should be minimal for pipe transfer. For the recertification of cleanrooms, the ISO14644-2 guideline must be followed, that is, once a year for ISO class 6 and every six months for ISO class 5.
Conclusion
All stages of cleanroom design, construction, testing and operation must be in accordance with internationally and or nationally accepted standards. In this article, some of the most important cleanroom standards including Federal Standard 209, ISO 14644, GMP guidelines and cleanroom classification were introduced.
Cleanroom Equipment by Ekeas
Cleanrooms include a variety of equipment, and in order for a cleanroom to meet standards, it must use the appropriate equipment. Here are a few examples of cleanroom equipment: